How RegCheck Works
From document upload to compliance report—our AI audit simulator guides you through every step of the regulatory review process.
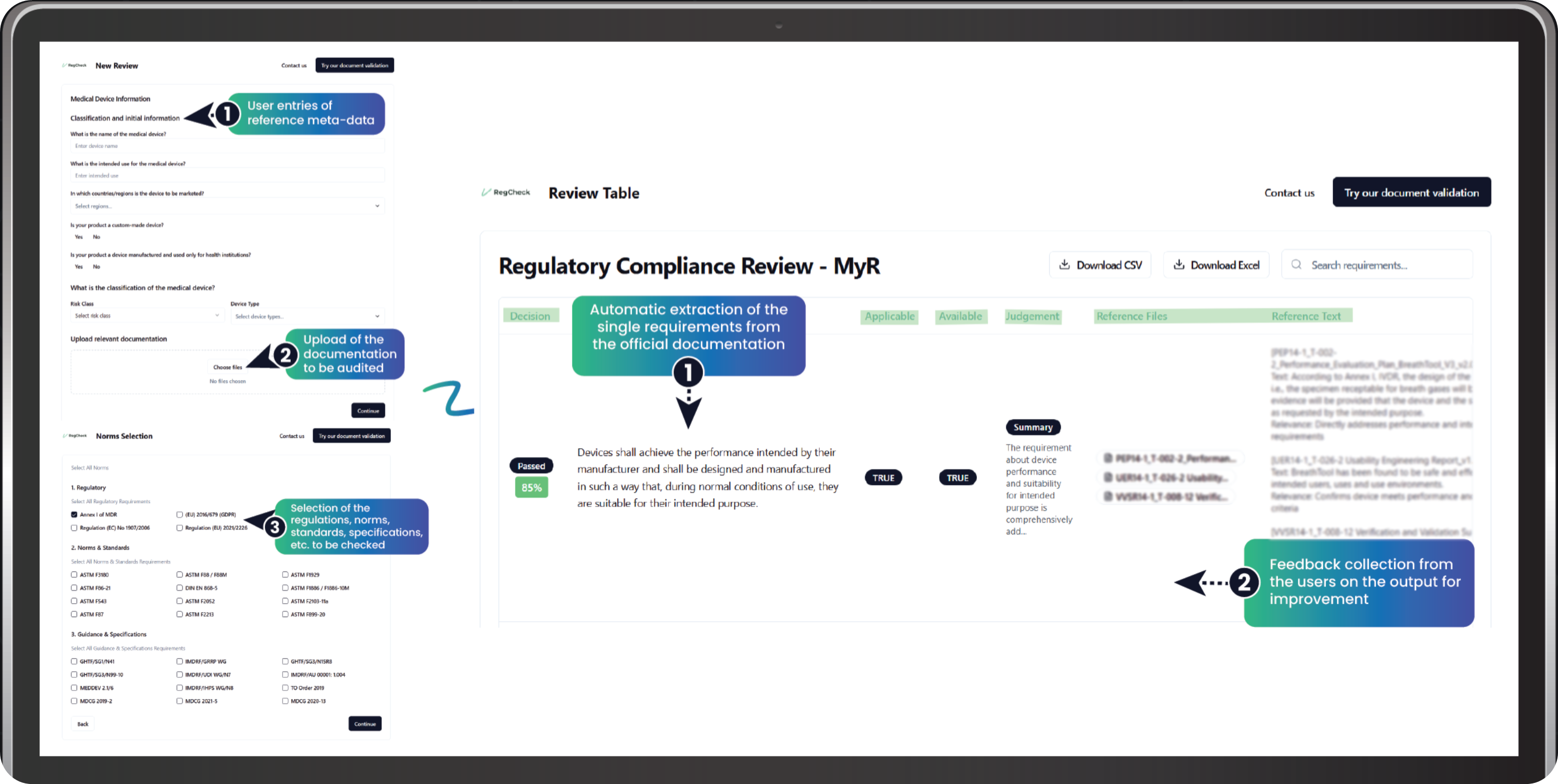
Your Path to Compliance
Enter Device Information
Start by providing reference meta-data about your medical device: name, intended use, target markets, device classification, and risk class. This helps our AI understand the regulatory context for your product.
Upload Your Documentation
Upload the regulatory documentation to be audited. You can submit documents at any stage—even early drafts—to identify gaps before they become costly revision cycles.
Select Applicable Standards
Choose the regulations, norms, standards, and specifications to check against: EU MDR annexes, ISO 13485, ISO 14971, and more. Our platform covers the full regulatory landscape for CE marking.
AI Reviews Your Documents
Our AI automatically extracts individual requirements from official documentation and screens your files against thousands of regulatory requirements—not just samples. It identifies gaps, inconsistencies, and missing elements that human reviewers often miss.
Review Compliance Results
Get a detailed Review Table showing each requirement, its applicability, availability in your documentation, AI judgment, and a summary explanation. Export results to CSV or Excel for further analysis. See your overall compliance score and exactly where to focus your efforts.
Continuous Improvement
Provide feedback on the AI's output to help improve accuracy over time. Our collaborative knowledge pool learns from your firm's expertise, creating increasingly precise assessments tailored to your specific context.
Why This Approach Works
Catch Issues Early
Submit drafts at any stage. Early gap identification prevents costly back-and-forth with Notified Bodies.
Exhaustive Coverage
AI reviews all requirements—not samples—catching inconsistencies that human reviewers often miss.
Transparent Decisions
Every judgment comes with reference files and explanatory text. No black-box decisions.
Export & Collaborate
Download results as CSV or Excel. Share findings with your team and Notified Bodies.
Ready to See It in Action?
Try our public instance or schedule a personalized walkthrough with our team.